sync2brain
And we make it even better …
Watch our new video!
Why we exist
Impact on Neuroscience
Impact on Medicine
Impact on Healthcare System
Our PRODUCTS
bossdevice MEDICAL
We are currently preparing the CE certification of the bossdevice MEDICAL as a medical device. Once certified, it may be used with compatible EEG amplifiers and compatible TMS stimulators for closed loop TMS experiments and therapy. Stay connected for updates!
In the meantime, bossdevice MEDICAL is available as an investigational device for approved clinical studies. If you are interested please contact us!


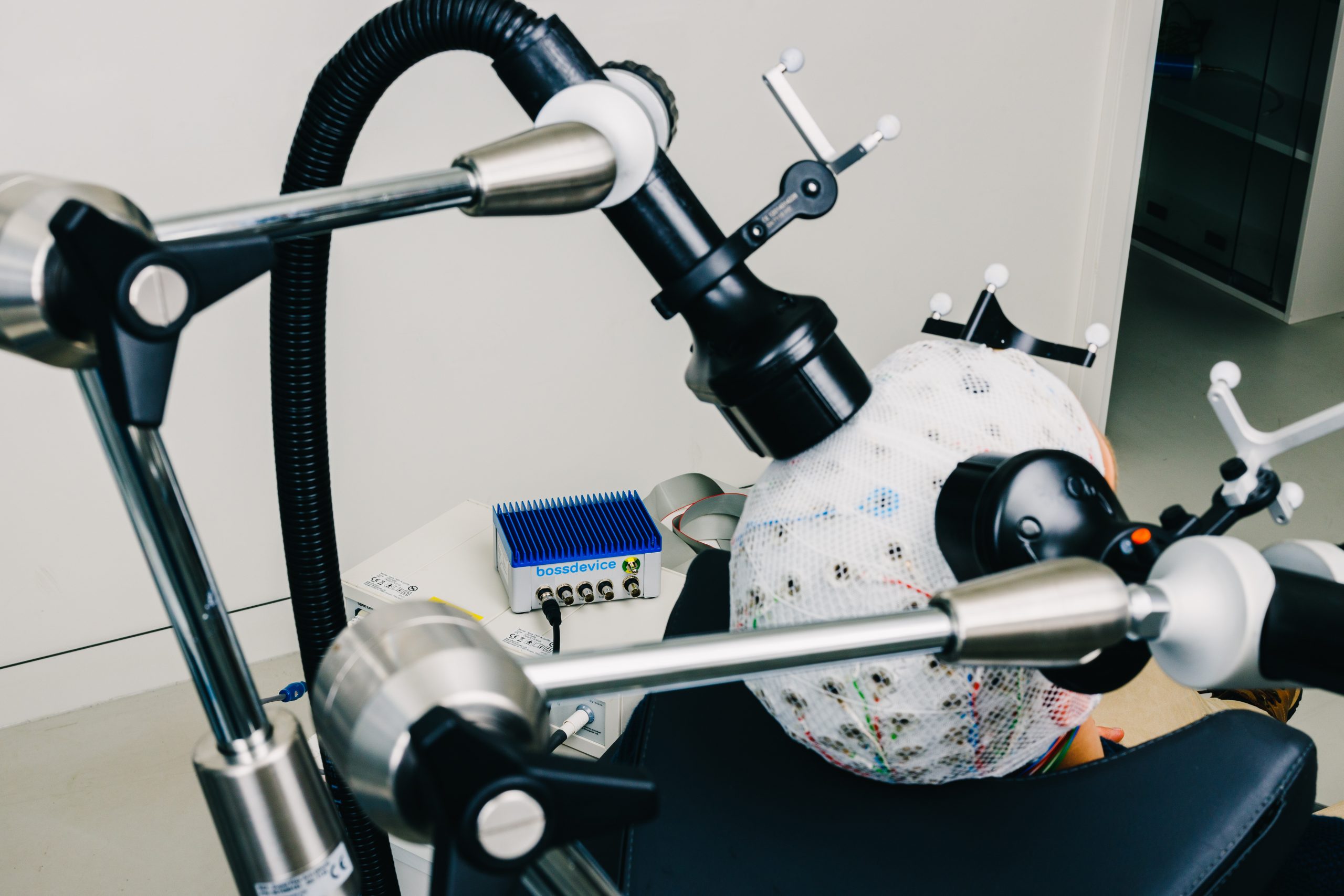
bossdevice RESEARCH
bossdevice RESEARCH is a real-time digital signal processor consisting of hardware and software algorithms. It is designed to read-in a real-time raw data stream from a bio- signal amplifier (electroencephalography, EEG), to continuously analyze this data and to detect patterns based on oscillations in different frequencies. When such a specific bio-signal pattern is detected, the device indicates this through a standard output port. This enables a connected device to know with millisecond accuracy when a specific bio-signal pattern occurs.
Next-Generation TMS
Clinical Studies
Research Labs
Patients
Grants in EUR

About US
Meet Ramona Samba, our CEO. In the video she explains who we are, what we do and where we are headed.
Meet our Team, …

Dr. Ramona Samba
CEO & Co-Founder
Head of Quality Assurance

Dr. Brigitte Zrenner
Clinical Studies & Co-Founder

Dr. Christoph Zrenner
CTO & Co-Founder

Henrike Stutzki
Head of Regulatory and Clinical Affairs

Christina Lemberg
Head of Quality Affairs

Pablo Romero Cumbreras
Technical Development

Simon Werner
Technical Development

Dr. Gabor Kozak
Technical Development and Senior Scientist

Ryan Rich
Customer Support

Rodrigo González O'Brien
Technical Development

You?
Message us on LinkedIn!
If you want to be part of our innovative team and want to improve the world of neurostimulation, message us on LinkedIn for an unsolicited application. We look forward to meeting you!
our Alumni

Hegel Nana

Samuela-Esther Aliman

Niko Mangold

Umair Hassan
Join Us!
Do you want to join a purpose driven company and community and become part of our team?
This is your chance!
purpose – yes.
responsibility – yes.
self-efficacy – yes.
room to grow – yes.
flexibility – oh yes.
Let’s talk!
We’re pleased to share that sync2brain has been selected for the Women TechEU program, an EU initiative supporting high-potential women-led deep-tech startups.
The program selected 40 companies out of more than 1,100 applicants, and we’re proud to be part of this cohort as we continue building and scaling our technology.


Frontier Research Competences for Neuro-modulation (FRESCO4NoPain)
sync2brain is an associated partner of the FRESCO4NoPain network.
FRESCO4NoPain is an EU-funded Marie Sklodowska-Curie Action, Doctoral Network that will provide world-class interdisciplinary training to 17 Doctoral Candidates in the area of non-pharmacological chronic pain treatment based on the pain-related brain oscillations and neuromodulation.
Do you want to be a part of the FRESCO4NoPain project?
Learn more by clicking here!
TMS in Stroke
Neurorehabilitation
TMS therapy is offered at the TMS clinic at the University Neurology Hospital of Tübingen, including clinical trials with sync2brain technology. Please see www.tms-nach-schlaganfall.de for details and to schedule an appointment for an evaluation. An application to the insurance company for reimbursement of the therapy cost is recommended.
Coverage in Media
Thanks to our Supporters …











